Abstract
Background: The WHO 2016 classification identifies myeloid malignancies with germline predisposition as a distinct subgroup (Arber DA et al., Blood 2016). We and other reported mutations of the DEAD-box RNA helicase 41 gene (g DDX41m) as the most common predisposition to MDS/AML (Sébert et al., Blood 2019). Relatively good outcomes have been suggested in small cohorts receiving heterogeneous treatment, but the prognostic significance of g DDX41m in AML patients (pts) treated with intensive chemotherapy (IC) has never been reported. Here, we analyzed the prognostic impact of g DDX41m in a large cohort of newly diagnosed AML pts treated with IC in 5 prospective ALFA (Acute Leukemia French Association) and FILO (French Innovative Leukemia Organization) trials.
Methods: We retrospectively screened 1690 AML pts (aged 18-85y) treated in ALFA0701 (EudraCT 2007-002933-36), ALFA0702 (NCT00932412), ALFA1200 (NCT01966497), ALFA1401 (NCT02473146) and LAM-SA (NCT00590837) clinical trials for DDX41 mutations using High Throuput Sequencing. DDX41 variants with a variant allele frequency (VAF) >40% were interpreted as causal if they were pathogenic or likely pathogenic by the American College of Medical Genetics and Genomics (ACMG) guidelines. The concurrence of a somatic DDX41 mutation was also considered as a strong evidence for the causality. Correlation between g DDX41m and covariates was realized using point biserial correlation and Fisher test for continuous and dichotomic variables, respectively. Allogeneic hematopoietic stem cell transplantation (HSCT) was considered as a time-depending variable, and all outcome analyses were stratified on the clinical trial.
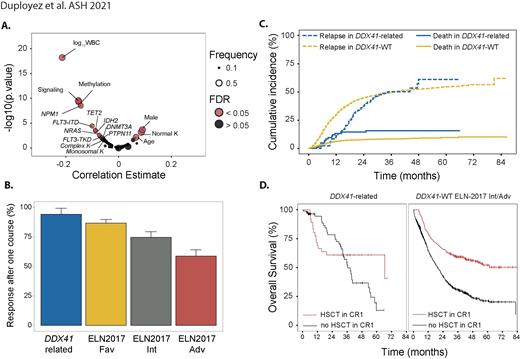
Results: We identified 86 unrelated pts with DDX41-related AML representing 5% of the whole cohort; 66 (77%) of them had additional somatic DDX41 mutations. Most common germline variants were p.D140fs (21%), p.M1? (8%), p.L283fs (7%) and p.K331del (5%); p.R525H and p.G530D/C/S accounted for 80% and 10% of all somatic mutations respectively. Compared to wild-type pts, DDX41-related AML were significantly older (65.5 vs 64y, p=0.036), with male predominance (74 vs 54%, p=0.002), had higher rates of normal karyotypes (77 vs 57%, p=0.006), lower WBC (2.0 vs 7.9 G/L, p<0.001), lower rates of NPM1 (4 vs 34%, p<0.001), FLT3-ITD (4 vs 22%, p<0.001), signaling (19 vs 53%, p<0.001) and DNA methylation related gene mutations (23 vs 57%, p<0.001) and had no complex karyotype (none vs 9%, p=0.009) (Figure 1A). According to ELN-2017, DDX41-related/DDX41-wt pts were favorable, intermediate and adverse in 4/36, 68/28, and 28/37% respectively.
After one induction course, CR/CRp was achieved in 81 (94%) DDX41-related AML compared to 1164 (73%) in DDX41-wt pts. In a multivariate analysis including WBC, ELN-2017 classification and clinical trial, presence of a g DDX41m was associated with significant higher CR/CRp achievement (OR, 5.39 [95% CI, 2.33-15.67]; p=0.0004) (Figure 1B).
After a median follow-up of 47.8 months, DDX41-related AML had a median OS of 39.7 (IQR, 19.4-66.4) months compared to 29.1 (IQR,10-not reached [NR]) months in DDX41wt (p=0.045). However, the prognostic impact on OS of g DDX41m was not independent of WBC and ELN-2017 classification (p=0.5). Relapse rates in DDX41-related pts were lower at 1-year (11 vs 30%), but then increased to join the relapse rates of DDX41-wt pts at 3 years (50 vs 50%, Figure 1C).
Finally, 35 DDX41-related and 288 non-favorable DDX41-wt pts received an HSCT in first CR. HSCT was associated with a prolonged OS in the non-favorable DDX41-wt cohort (HR, 0.61 [95% CI, 0.49-0.76]; p<0.001) but not in the DDX41-related pts (HR, 0.768 [95% CI, 0.35-1.68]; p=0.5, Figure 1D). When considering relapse and death as competitive risks, DDX41-related pts showed an increase of transplant-related mortality (TRM) within the first year (13% vs 6%, p=0.026). However, HSCT was associated with a prolonged relapse-free survival (HR, 0.42 [95% CI, 0.21-0.88]; p = 0.02) in this group.
Conclusion: This is the first study evaluating the prognostic impact of g DDX41m in a large cohort of AML pts prospectively treated with IC. DDX41-related AMLs represented a rare specific entity and were associated with a higher response rate, prolonged time to relapse without independent OS advantage compared to DDX41-wt AML. These results suggest that consolidation/maintenance strategy might be adapted in these pts.
Lambert: ASTELLAS: Consultancy; CELGENE/BMS: Consultancy. de Botton: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Forma Therapeutics: Honoraria, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Syros: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pierre Fabre: Other; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees. Recher: Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MaatPharma: Research Funding; Macrogenics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Pigneux: Amgen: Consultancy; Sunesis: Consultancy, Research Funding; BMS Celgene: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Dombret: Amgen: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Novartis: Research Funding; Pfizer: Honoraria, Research Funding; Servier: Research Funding; Abbvie: Honoraria; BMS-Celgene: Honoraria; Daiichi Sankyo: Honoraria. Delabesse: Astellas: Consultancy; Novartis: Consultancy. Sebert: Abbvie: Consultancy; BMS: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal